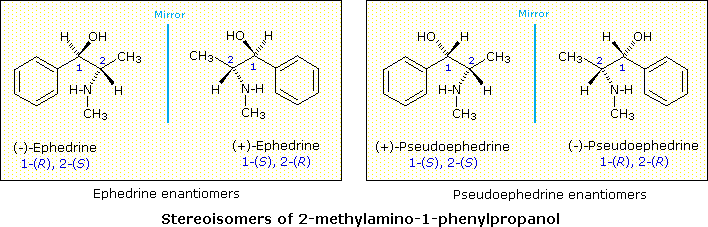
The
natural molecule 2-methylamino-1-phenyl-1-propanol has 2 chiral centers and is
derived from the Chinese shrub Ma Huang. The shrub contains 2 physiologically
active compounds ephedrine and pseudoephedrine-both of which are stereoisomers
of the molecule 2-methylamino-1-phenyl-1-propanol. Each compound has an
enantiomer, which isn’t found in the natural shrub but can be synthesized. The
two active compounds can not be enantiomers as they have such different
properties. Ephedrine has a melting point of 35-40*c and pseudophedrine has a
melting point of 119*C. Pseudoephedrine, in particular, can be used as a decongestant and the salts pseudoephedrine hydrochloride and pseudoephedrine sulfate help to relieve nasal/sinus problems-it can be found in many
common prescription or over-the-counter drugs such as Sudafed. Pseudoephedrine
works by shrinking the blood vessels in the nasal passage as this is what can
cause a blocked nose. Some ADHD patients have also been prescribed
pseudoephedrine due to its properties as a stimulant. The drug, along with many
others, works by having a calming effect on the patient.
Thursday, October 31, 2013
Stimulating Ephedrine/Pseudoephedrine!
"Ma Huang" is a Chinese shrub that has been used for centuries in traditional Chinese medicine as an anti-asthmatic and stimulant. This particular shrub contains not one, but TWO coumpounds that have 2 chiral centers - Ephedrine and Pseudoephedrine. Both these compounds are stereoisomers of 2-methylamino-1-phenyl-1propanol and are both optically active. The structural formula of 2-methylamino-1-phenylpropanol has two stereogenic carbons shown at Carbon #1 & #2. Each stereocenter can have an R or S configuration, so based on the general rule - a structure having n chiral centers will have 2^n possible combinations - there are 4 possible stereoisomers. In addition, these particular compounds exists as diastereomers to each other - Ephedrine is a diastereomer to pseudoephedrine.
In addition to the aforementioned uses, both ephedrine and pseudoephedrine can relieve hypotension - but pseudoephedrine has less effect. Other uses include weight loss promotion, nausea relief, and to treat bronchitis.
References:
Vitamin E, 4 Chiral Centers
VITAMIN E

Vitamin E is a molecule with four chiral centers. 2R and 2S
When reading about Vitamin E, I was shocked to find that it has a wide variety of uses. It mainly is used to keep organs functioning properly and at their highest potential. It can be found in many foods and it is rare for someone to have a Vitamin E deficiency but supplements are available. These supplements are often given to patients who are receiving aggressive medication in order for the side effects to be lessened.
References:
http://commons.wikimedia.org/wiki/File:Tocopherol,_alpha-.svg
http://www.webmd.com/vitamins-supplements/ingredientmono-954-VITAMIN%20E.aspx?activeIngredientId=954&activeIngredientName=VITAMIN%20E
Two Chiral Centers: Oxytocin
Oxytocin
Oxytocin is colloquially referred to as the "bonding hormone." It typically works in conjunction with vasopressin (cys-tyr-phe-gln-asn-csy-pro-arg-gly-NH2) and only differs by two amino acids. Oxytocin and vassopressin are released by the human posterior pituitary gland and are the only known hormones to act at a distance from the brain.
Peripherally it is known to act at the mammary glands causing milk to be "let down" during lactation, in the uterus during the birthing process causing the cervix to dilate and at wound sites by decreasing some cytokines and has been observed to improve wound healing after positive social interactions. Locally, in the brain, it plays a role in sexual arousal, social bonding, maternal behavior and romantic attachment.
It has been theorized that in its reduced form, oxytoceine, it may act as a free radical scavenger, donating electrons to free radicals, making them more stable and therefore less likely to cause biological harm. Synthetic oxytocin has been used to induce labor and it has been suggested that it might help people who suffer from social anxieties, mood disorders and autism. Almost all vertebrates have an oxytocin-like hormone.
http://en.wikipedia.org/wiki/Oxytocin
Ketamine
Ketamine, we have all probably heard it mentioned on TV but
what exactly is it? Ketamine is classified as a NMDA receptor antagonist and consist
of a chlorophenyl and cyclohexanone group. It is commonly used in medicine as
an anesthesia supplement; however it is also used in emergency medicine for
pain relief, and in some cases for bronchospasms. Ketamine affects the body by
increasing blood pressure, heart rate, does cause hallucinations for some
individuals, however it does not suppress the respiratory system as much as many
other anesthesia drugs. This is another reason why this drug is great for
emergency medicine because there is not as much concern over the airway,
although with any drug airway still needs to be monitored accordingly. This drug
is also a good choice for patients whom just suffered a traumatic injury and
are in need of immediate surgery; because usually these patients will have lost
a great amount of blood volume and by administering ketamine it will help increase
the blood pressure back to normal levels. However while administering ketamine
will help with increasing the blood pressure, IV fluids will still need to be
administered rapidly to increase it.

Isoleucine
Isoleucine is an amino acid. This amino acid cannot be made by humans so it has to be ingested. The codons that code for this amino acid are AUU AUC AUA.
Four sterioisomers of isoleucine are possible, but it is only found as 2S,3S)-2-amino-3-methylpentanoic acid
Isoleucine can also be made to use glucose.
D-Ribulose
Monosaccharides or simple sugars are aldehyde or ketone derivatives of straight-chain polyhydroxy alcohols containing at least 3 carbon atoms. D-Ribulose is one of the example of monosaccharides which cannot be hydrolyzed to form sompler saccharides. Ribulose sugars are composed in the 5 membrane phosphate pathway. It is important in the formaiton of many bioactive substances. D-ribulose is an intermediate in the fungal pathway for D-arabitol production. The streochemistry of both carbon 3 and 4 are R.
.
Voet, Donald and Voet, Judith G. Biochemistry
|
THC: is it for me?
Tetrahydrocannabinol, or THC for short, is a chiral molecule with 2 stereocenters. It is the main psychoactive constituent of cannabis, or marijuana. Cannabinoids are compounds that have a similar structure to THC. Marijuana has been in the news a lot lately with certain states legalizing specific small amounts and it being medically prescribed by some physicians. Some medical benefits include: pain relief, appetite stimulation (in AIDS wasting syndrome and anorexia), for glaucoma, epilepsy, easing the spasticity seen in MS and spinal cord injuries, and control of nausea and vomiting in chemo patients - just to name a few. THC also produces psychoactive effects.
THC is a lipophilic molecule. It acts on G-protein coupled receptors, decreasing cAMP and in turn inhibiting adenylate cyclase. Some studies believe that THC has anticholinesterase action, meaning it could possibly be a form of treatment for Alzheimer's disease! Maybe Bob Marley knew what was up.
references:
Organic Chemistry at Work
When taxol is given as an injection, it is a thick, clear to yellow solution that is administered intravenously in hopes of decreasing the size of a tumor. It is commonly used in treated breast, ovarian, lung, and brain cancer. It can also be used in situations related to AIDS-related Kaposi's sarcoma. Kaposi's sarcoma is a tumor that is caused by the human herpesvirus 8 (HHV8), and it is found in the skin and mucous membranes. In certain patients populations, Taxol is also used for: cancer of the bladder, cancer of the cervix, cancer of the endometrium, cancer of the esophagus, cancer of the fallopian tube or lining of the abdomen (spreading from the ovary), cancers of the head and neck, prostate cancer, stomach cancer, cancer of the testes, and cancers with an unknown primary site.
Paclitaxel itself however, is a white to off-white crystalline powder with the molecular formula C47H51NO14 and a molecular weight of 853.9. It is very lipophilic, meaning that is is easily soluble in fats and lipids, and insoluble in water. It also has a melting point of around 216-217° C.
Taxol was first extracted in 1971 from the bark of the Pacific Yew, a conifer found in the Northwest of the United States.
Overall, I find Taxol to be a fascinating organic compound since so many of my patients use it as a form of chemotherapy and it is directly involved in my work.
http://www.drugs.com/cons/taxol.html
http://en.wikipedia.org/wiki/Taxus_brevifolia
http://en.wikipedia.org/wiki/Kaposi%27s_sarcoma
http://www.rxlist.com/taxol-drug.htm
Chapter 3: Stereochemistry & Chirality. http://courses.chem.indiana.edu/c341/documents/C341S2010CH3Chiralitystudent.pdf
http://www.youtube.com/watch/?v=-O0lsJywFFg
Glucose
Glucose C6H12O6, also known as D-glucose or dextrose is a simple monosaccharide found in plants and necessary for our survival. It is absorbed directly into the bloodstream during digestion. Cells use it as a secondary source of energy and a metabolic intermediate. Glucose is one of the main products of photosynthesis and it is a main fuel for cellular respiration. Glucose exists in several different molecular structures, but all of these structures can be divided into two families of mirror-images (stereoisomers). Only one set of these isomers exists in nature, those derived from the "particular chiral form" of glucose, denoted D-glucose.
Glucose is very important in our own diets and it is especially important for diabetic individuals to be aware of their intake of it. For instance if someone is having a diabetic emergency and it hypoglycemic, the best thing to do is to have them ingest glucose tablets or gels. Having something like a sugary drink or candy will not work as fast because it is an alternate form of sugar and must first be broken down into glucose before it can be properly used in the blood stream. Too much glucose is also a issue especially when your bodies insulin response is abnormal.
The chemical D-glucose is sometimes referred to as dextrose, a historical name that derives from dextrorotatory glucose because a solution of D-glucose in water rotates the plane of polarized light to the right (dextro).[3] However, the D- in D-glucose refers to a chiral chemical similarity property in sugars, not the property of rotating light (for example, D-fructose rotates light to the left). For this reason, the D- and L- designations in sugars do not perfectly predict optical rotation, and do not refer to this property.
Glucose or D - Glucose has a total of four chiral centers with the following stereochemistry:
http://www.chemspider.com/Chemical-Structure.96749.html
http://en.wikipedia.org/wiki/Glucose
Glucose is very important in our own diets and it is especially important for diabetic individuals to be aware of their intake of it. For instance if someone is having a diabetic emergency and it hypoglycemic, the best thing to do is to have them ingest glucose tablets or gels. Having something like a sugary drink or candy will not work as fast because it is an alternate form of sugar and must first be broken down into glucose before it can be properly used in the blood stream. Too much glucose is also a issue especially when your bodies insulin response is abnormal.
The chemical D-glucose is sometimes referred to as dextrose, a historical name that derives from dextrorotatory glucose because a solution of D-glucose in water rotates the plane of polarized light to the right (dextro).[3] However, the D- in D-glucose refers to a chiral chemical similarity property in sugars, not the property of rotating light (for example, D-fructose rotates light to the left). For this reason, the D- and L- designations in sugars do not perfectly predict optical rotation, and do not refer to this property.
Glucose or D - Glucose has a total of four chiral centers with the following stereochemistry:
http://www.chemspider.com/Chemical-Structure.96749.html
http://en.wikipedia.org/wiki/Glucose
Chiral Terpenes
There are many chiral compounds found in nature. One of the most diverse and common chiral compounds found in nature is called terpenes. Terpenes is a common organic compound found in a wide variety of plants, especially conifer resins, sometimes certain insects can produce the chemical as well. Because of their aromatic hydrocarbons, terpenes have been sought after for many things such as beer, food, aromatic oils, etc.
There are many different terpenes chemicals, but for this blog, the one that will be used in this example is Limonene. Limonene is a cyclic terpenes that is a colorless liquid. It is commonly used in cosmetic products as well as being the main odor constituent of citrus. It is also used in food manufacturing and some medicines, such as a flavoring to mask the bitter taste of alkaloids, and as a fragrant in perfumery. Limonene is also used as botanical insecticide, particularly the (R)-(+)-enantiomer. It is also added on to cleaning products such as hand cleansers to give a lemon-orange fragrance. In natural and alternative medicine, Limonene is sold to relieve gastroesophageal reflux disease and heartburn as well.

http://en.wikipedia.org/wiki/Limonene
http://www.nky-kk.co.jp/english/products.htm
There are many different terpenes chemicals, but for this blog, the one that will be used in this example is Limonene. Limonene is a cyclic terpenes that is a colorless liquid. It is commonly used in cosmetic products as well as being the main odor constituent of citrus. It is also used in food manufacturing and some medicines, such as a flavoring to mask the bitter taste of alkaloids, and as a fragrant in perfumery. Limonene is also used as botanical insecticide, particularly the (R)-(+)-enantiomer. It is also added on to cleaning products such as hand cleansers to give a lemon-orange fragrance. In natural and alternative medicine, Limonene is sold to relieve gastroesophageal reflux disease and heartburn as well.

http://en.wikipedia.org/wiki/Limonene
http://www.nky-kk.co.jp/english/products.htm
Taxol
Taxol is a natural product that is used in cancer treatment.
It's generic name is Paclitaxel. It is used as a chemotherapy drug and it is
labelled as a "plant alkaloid." It has been used to treat many
cancers including breast, ovarian, lung, bladder, prostate, melanoma, and
esophageal cancers. It is often used against cancers that have a solid tumor.
Taxol gives rise to many of the usual side effects that accompany
chemotherapy.
Taxol is a plant alkaloid which is made up of two parts,
vinca alkaloids and taxanes. The vinca alkaloids are made from the periwinkle
plant and the taxanes are derived from the bark of the Pacific Yew tree. This
became an environmental concern because the trees have to be destroyed in order
to manufacture the compound. It
interferes with cell division by affecting the decomposition of microtubules.
I decided to pick Taxol for this entry because I feel that
all cancer research is relevant to chemistry and our lives.
Sources:
http://chemocare.com/chemotherapy/drug-info/Taxol.aspx#.UnL_SRDli8A
http://en.wikipedia.org/wiki/Paclitaxel
I'll Make You A Man
Testosterone is a hormone in the body that almost everyone is familiar with. It is responsible for the secondary sexual traits that are seen in males. It is produced in the testes and adrenal cortex in males. It is also produced in females but in smaller amounts. In females it is produced in the ovaries and also the adrenal cortex. This is a very important hormone in the medical field and even psychological field.
The testosterone molecule has six different chiral centers which means it can have 64 different stereoisomers. It has one enantiomer and 62 diastereomers.
The testosterone molecule has six different chiral centers which means it can have 64 different stereoisomers. It has one enantiomer and 62 diastereomers.
Works Cited
Magolan, J. "Chem 275 Practice Problem Set." . N.p., 01 10 2010. Web. 31 Oct 2013. <http://webpages.uidaho.edu/chem275/files/Chem 275 Practice Problem Set - Weeks 5 and 6 - Solutions.pdf>.
Google Search-Testosterone Definition
Goo
tes
Morphine
is used predominantly as a painkiller and to develop other, similar painkillers. Morphine was named for the Roman god of
dreams and sleep, presumably due to its numbing effect on pain and mood and the
sleep it often induces. Morphine can
also, unfortunately, causes digestive upset including nausea, vomiting, and
constipation, along with other side effects like seizures and a slowed
heartbeat. It is also extremely
addictive, as are compounds derived from it, the so-called opiods. Why opiods?
Morphine (and some similar compounds) come from Papaver somniferum, the opium poppy, though it can also be synthesized
in a lab. Morphine has five chiral
centers (highlighted in red in the two-dimensional drawing below).
Sources:
images from Wikipedia, red highlights on 2D structure mine
Subscribe to:
Posts (Atom)